Salt Crystallization and Concentration

Salt Crystallization and Concentration
Application Examples:
Process Role
Evaporation and concentration
Heating salt solutions to supersaturation for crystallization.
Cooling crystallization
Controlling temperature to precipitate solutes (e.g., Glauber’s salt cooling).
KDP PHE Selection
Anti-scaling design
Wide flow channels or embossed patterns to reduce clogging.
Materials
316L stainless steel (chloride-resistant) or nickel-based alloys (ammonium salt-resistant).

Salt Crystallization & Concentration Process Flow
Typical Salts Produced: NaCl, Na₂SO₄, NH₄Cl, KCl
Here’s a detailed step-by-step explanation of the salt crystallization and concentration process, including equipment used (with emphasis on plate heat exchangers) and key technical parameters:
Key Stages:Feed Preparation
Raw brine
(e.g., seawater, salt lake brine, or industrial wastewater).
Filtration: Remove suspended solids (sand, organics) via sand filters or centrifuges.
Chemical Treatment: Add NaOH/Na₂CO₃ to precipitate Ca²⁺/Mg²⁺ (softening).
Clarified brine (20–25% salt concentration).
Plate heat exchangers (PHEs) preheat brine to ~60–80°C using waste heat.
Key Stages:Evaporation Concentration
Increase salt concentration to near saturation (e.g., ~28% for NaCl).
Multi-effect evaporation (energy-efficient) or mechanical vapor recompression (MVR).
Brine enters 1st-effect evaporator (heated by steam at ~120°C).
Water evaporates; concentrated brine flows to next effect at lower pressure/temperature.
Final concentrate exits at ~40–50% salt (super-saturated).
Role of Plate Heat Exchangers
PHEs transfer heat from steam/live vapor to brine.
Recover heat from vapor condensate.
NaCl/KCl: 316L stainless steel PHEs.
NH₄Cl/Na₂SO₄: Titanium or nickel-alloy PHEs (anti-corrosion).
Key Stages:Crystallization
Form uniform salt crystals from super-saturated brine.
Forced-circulation crystallizer or Oslo-type crystallizer.
Super-saturated brine enters crystallizer vessel.
Seeding: Add fine salt crystals to initiate controlled growth.
Cooling: Plate heat exchangers maintain ~30–50°C (for cooling crystallization) or ~60–100°C (for evaporative crystallization).
Crystals grow to 0.2–1.0 mm size.
PHE Application:
Chilled water circulates through PHEs to remove latent heat.
Wide-gap plates or pulsed flow to prevent fouling.
Key Stages:Separation & Drying
Separate crystals from mother liquor (residual brine).
Screen-scroll centrifuges (moisture reduced to 3–5%).
Spray with fresh water to remove impurities (optional).
Fluidized-bed dryers (hot air at ~150°C) for final moisture <0.5%.
Sized crystals stored in silos or bags.
PHEs recycle waste heat from dryer exhaust air to preheat incoming brine.
Process Flow Diagram (Simplified)
↓
↓
↓
↓
↓
↓

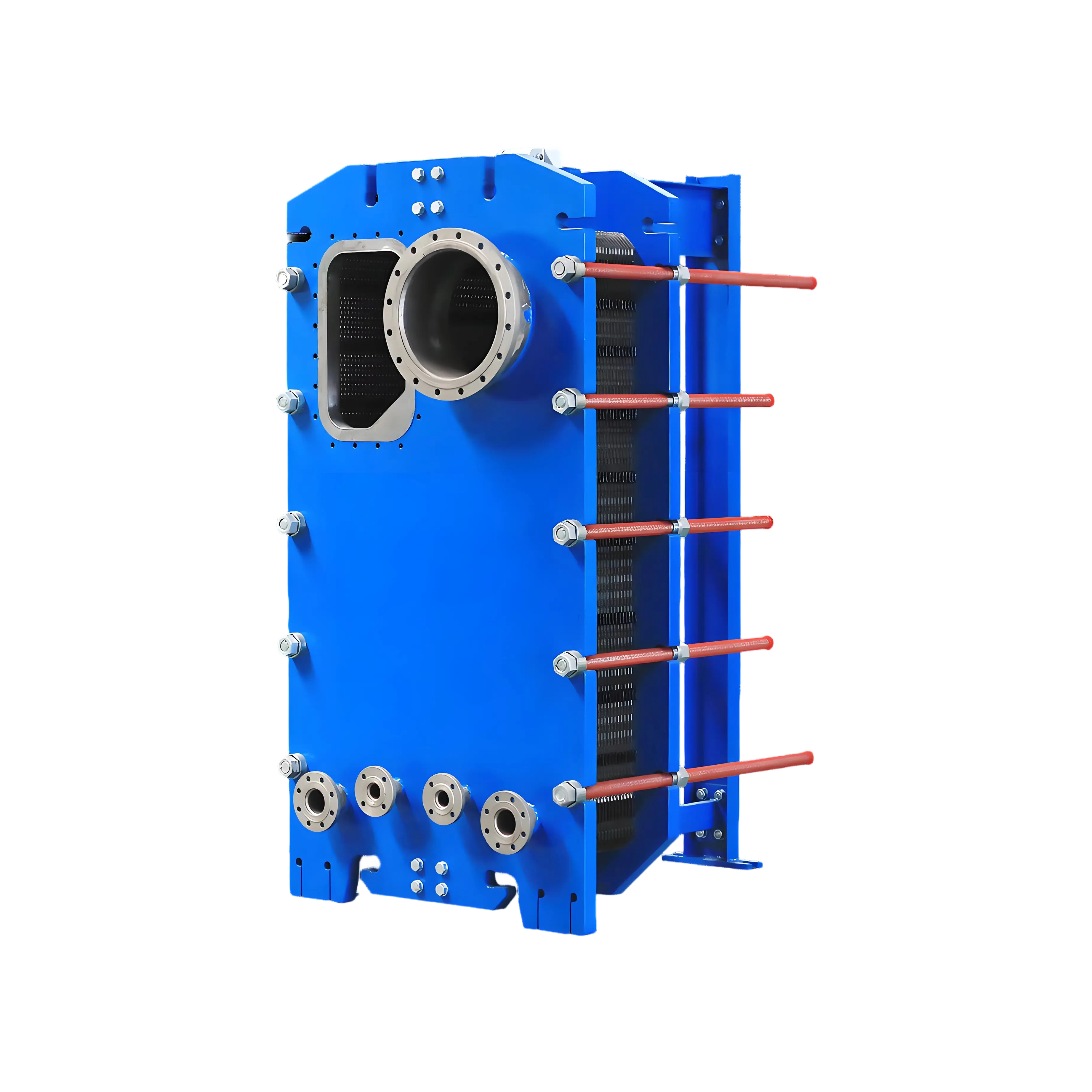
Role of KDP Plate Heat Exchangers (PHEs)
Recovers heat from condensate/exhaust streams (30% energy savings).
Uses steam/vapor to raise brine temperature.
Controls supersaturation by precise temperature adjustment.
Detachable plates: For cleaning scale (CaSO₄, Mg(OH)₂).
Electropolished surfaces: Reduce crystal adhesion.
Key Technical Parameters
Comparison of Crystallization Methods
Optimization Tips
Scale Prevention
Use pulsed flow or ultrasonic PHEs to disrupt scaling.
Material Selection
NaCl: 316L stainless steel.
Ammonium salts: Titanium or Hastelloy.
Automation
Control supersaturation via real-time temperature monitoring.

King DuPont, China famous brand of heat transfer and fluid handling supply platform.
Contact

Get Free Quotes
NEED TO CHAT?
We will get back to you within 24 hours of receiving the message.

