Gas Treatment——Ammonia Condensation Chlorine Cooling

Gas Treatment ——Ammonia Condensation, Chlorine Cooling
Application Examples:
Process Role
Ammonia condensation
Converting gaseous ammonia into liquid ammonia (below -33°C).
Chlorine cooling
Lowering the temperature of hot chlorine gas from electrolysis (preventing equipment corrosion).
KDP PHE Selection
Low-temperature suitability:
Special sealing materials (e.g., fluororubber).
Corrosion resistance:
Titanium (resistant to wet chlorine corrosion).
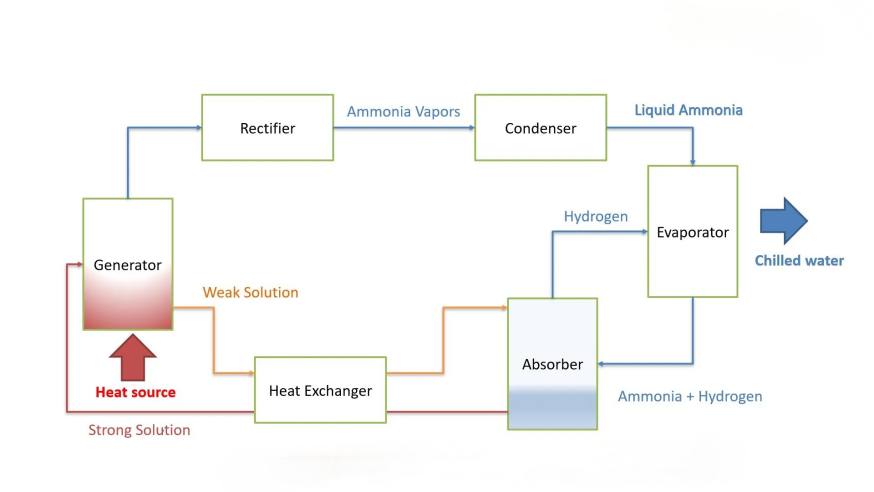
Detailed Process of KDP Plate Heat Exchangers in Ammonia Condensation
and Chlorine Cooling
Plate heat exchangers are widely used in ammonia condensation and chlorine cooling, two critical processes in the chemical industry. However, due to the differing properties of ammonia and chlorine (e.g., corrosivity, toxicity, phase-change behavior), their process flows and design requirements vary significantly. Below is a detailed breakdown:
1. Ammonia Condensation -Ammonia Synthesis or Refrigeration Systems
Process Flow
High-temperature gaseous ammonia (70–120°C, 1–2 MPa) from the synthesis reactor or compressor enters the PHE’s gas channel.
If lubricants or impurities are present, a oil separator is used for pretreatment.
Cooling water (or chilled water) flows countercurrently on the opposite side (typically 20–30°C, depending on cooling tower capacity).
Turbulent flow enhances heat transfer.
Ammonia gas releases latent heat on the plate surface, condensing into liquid ammonia (saturation temperature depends on pressure).
Condensate collects at the bottom outlet and flows to an ammonia storage tank or evaporator (in refrigeration systems).
If gases (e.g., H₂, N₂) are present, a vent valve or purge system is installed at the PHE’s top.
Key Design Parameters
Plates: 304/316 stainless steel (resists ammonia but must avoid chloride stress corrosion).
Gaskets: EPDM or NBR (resistant to ammonia swelling).
High-NTU (Number of Transfer Units) plates (e.g., herringbone pattern) for efficient condensation.
Wide gas channels prevent condensate accumulation and excessive pressure drop.
2. Chlorine Cooling -Chlor-Alkali Industry or Chlorine Processing
Process Flow
Wet chlorine gas (80–100°C, containing traces of HCl and moisture) from electrolysis enters the PHE.
Sulfuric acid scrubbers remove moisture to prevent hydrochloric acid formation.
Chlorine passes through titanium or graphite PHEs, cooled to 40–50°C by water.
Maintain slight positive pressure (0.1–0.3 MPa) to avoid air ingress (explosive mixtures).
For liquefaction, brine (-15 to -10°C) further cools chlorine to below -10°C, partially liquefying it.
Gas-liquid mixture enters a separator; liquid chlorine is stored, while uncondensed gas is recycled.
Chlorine leak detectors and NaOH scrubbers neutralize accidental releases.
Key Design Parameters
Plates: Titanium or titanium-palladium alloy (resists wet chlorine; stainless steel is prohibited).
Gaskets: PTFE or fluoropolymer (Viton).
Wide-gap plates or chemical cleaning if chlorine contains particulates (e.g., salt mist).
Coolant outlet temperature must stay above chlorine’s dew point to avoid HCl condensation.
Comparison Summary

King DuPont, China famous brand of heat transfer and fluid handling supply platform.
Contact

Get Free Quotes
NEED TO CHAT?
We will get back to you within 24 hours of receiving the message.

